Coagulation
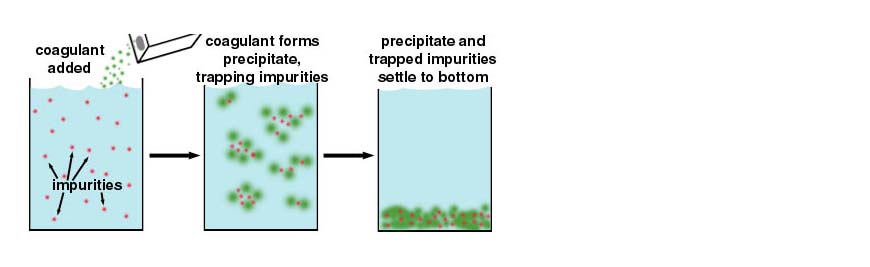
Solids are removed by sedimentation (settling) followed by filtration. Small particles are not removed efficiently by sedimentation because they settle too slowly; they may also pass through filters. They would be easier to remove if they clumped together (coagulated) to form larger particles, but they don't because they have a negative charge and repel each other (like two north poles of a magnet).
In coagulation, we add a chemical such as alum which produces positive charges to neutralize the negative charges on the particles. Then the particles can stick together, forming larger particles which are more easily removed.
In coagulation, we add a chemical such as alum which produces positive charges to neutralize the negative charges on the particles. Then the particles can stick together, forming larger particles which are more easily removed.
The coagulation process involves the addition of the chemical (e.g. alum) and then a rapid mixing to dissolve the chemical and distribute it evenly throughout the water.